NHS urged to contact patients with ‘modular neck’ implants who are unknowingly at increased risk of cobalt poisoning

An NHS hip replacement patient was poisoned and left in agony by an implant used in thousands of other operations, she has revealed.
Tracey O’Neill, 56, spent years struggling with memory loss and paying to see private cardiologists because of side effects that have since been linked to cobalt poisoning caused by her replacement hip.
About 5,000 patients in Britain had the same “modular neck” hips made with metal parts implanted between 2009 and 2017. The false hips are proven to have higher failure rates, putting anyone with them at increased risk of cobalt poisoning.
Mrs O’Neill, a former fitness instructor and long-distance runner, has been in agony since having both hips replaced with the implants – the first of which was when she was 46.
She said she now “feels trapped in a 90-year-old’s body”.
She told The Telegraph that her life had been “taken from her” but no one, including the NHS, would recognise the harm caused or accept responsibility.
“I’m just trying to survive every day,” she said. “I’d be lying if I didn’t say that I feel that my life has been taken from me and I am just existing.”
The implants have not been recommended for use on the NHS since 2017 after it emerged that wear and tear could lead them to leak particles of cobalt into a patients’ blood streams. This can be deadly and lead to heart failure, memory loss, depression, nerve damage, sight loss and deafness, among other issues.
Campaigners and charities representing patients have demanded an investigation from the Medicines and Healthcare products Regulatory Agency (MHRA), and for the NHS to notify all patients who could be affected.
They fear the NHS has not done enough to track down those who had the implants so that they could be monitored or offered replacements.

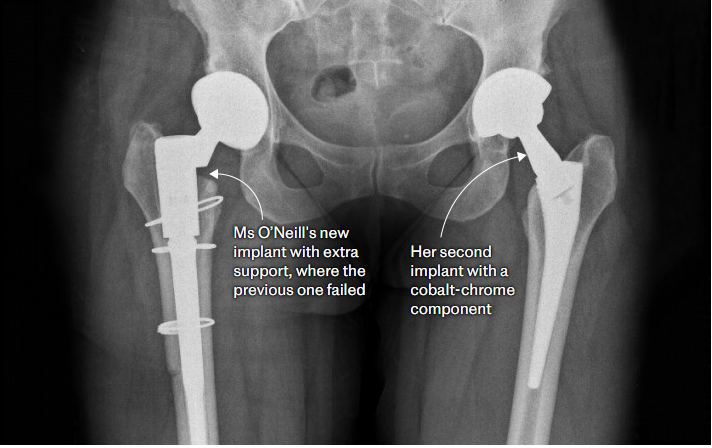
Mrs O’Neill had her right hip replaced at the Hospital of St Cross, run by the University Hospitals Coventry and Warwickshire NHS Trust (UHCW), in 2014.
She was never informed about what implant was being used, but the “modular neck” was the hospital’s product of choice for younger patients because of its apparent benefits. A year later, the same product was used in her left hip by the same surgeon privately.
UHCW stopped using it in 2016 because of fears of its “noxious effects” on patients, the chief executive admitted in a letter seen by The Telegraph.
Mrs O’Neill, like other patients with the implants, should have been regularly monitored for poisoning but was not. Her hip surgeries and the products used were not recorded on the National Joint Registry (NJR), despite this being mandatory in order to track the performance of implants, surgeons and hospitals.
She started to experience symptoms within two years of surgery – including pain, redness and swelling – but the hospital made no connection. By 2019, Mrs O’Neill was suffering from shortness of breath and fatigue, she had also put on weight and was seeing a heart specialist.
Mrs O’Neill’s condition continued to deteriorate despite years of seeking help, and it was not until 2020 when she saw a surgeon at a private clinic that the damage done by both the hip implants and the cobalt poisoning came to light.
“I got to the point that I couldn’t even, in a basic yoga class, I couldn’t get down and up from the floor,” she said. “I started to feel I had this horrible metal taste in my mouth. I started to lose my memory.

“I would be in meetings, and I would just forget the next word. People would think that I had just lost my marbles. I couldn’t understand what was going on, but I knew something wasn’t right. I didn’t feel well. I didn’t look well.”
Mrs O’Neill’s right hip had fractured, poisoning her with cobalt, and she underwent private revision surgeries to get it replaced. The second cobalt hip is still being monitored by the NHS, but Ms O’Neill says her hospital has continually cancelled appointments, and she has not had so much as a blood test in more than a year. She relies on walking sticks and lives in constant pain.
She said: “Is it normal to be in a relationship but you can’t sleep in the same bed? Is it normal to not be able to go out for dinner in the evening because you’re so tired and your body is in pain? That you’re scared to go into a restaurant because you don’t know what sort of chair is going to be in there, or if the toilet is going to be upstairs or downstairs?”
The mother of one fears there are thousands of people who do not know they are at risk because it was not standard practice to inform patients about the implant they would be receiving.

- The friction from wear and tear of the implant can cause the hip to fracture, releasing metal ions into the surrounding tissue
- The release of cobalt particles is particularly dangerous, it can poison tissue, turning it black and enter the blood stream traveling to other parts of the bodyThe modular neck hips were innovative when they were introduced in the noughties because they had three artificial joints, which gave surgeons greater flexibility to tailor the implants to individual patients for a better fit, while also being less invasive and preserving more healthy tissue.
However, the gradual wear and tear of the cobalt-chrome neck against the other parts caused metal particles to leak into the blood and the products to fail sooner, particularly if it was paired with titanium.
Various manufacturers created such designs but they have been used less frequently as awareness of the dangers has grown. They are not banned, and surgeons can use their discretion to decide it is suitable for certain patients, such as a very active younger person, because of the durability despite the risks.
Experts estimate that about 100,000 people have had such a hip implanted globally, including in the US, where lawsuits against manufacturers are ongoing.
The modular neck hips were in “general use” on the NHS between 2009 and 2017, and recommended for younger patients because of the alleged benefits. They remain on the market alongside alternative versions where cobalt-chromium is paired with other materials less likely to degrade, such as ceramic.
It comes just over a decade since the Telegraph exposed a separate metal hip scandal which involved two-piece metal implants that have been phased out since 2012 except for use in exceptional cases.
Cobalt is still commonly used in the 100,000 hip replacements carried out each year in the UK, but the growing fears of its toxicity has led a number of surgeons to stop using it altogether.
Prof Alister Hart, a leading consultant hip surgeon and chair of orthopaedics at University College London, called for surgeons “to avoid cobalt-chromium wherever possible” in hip implants.
He said it was “vulnerable to corrosion when paired next to itself or titanium” and “creates a battery with the body’s fluid”.
“The sad fact is that 99 per cent of patients with corroding implants could have avoided problems, because there are several alternatives to cobalt-chromium.”
Prof Colin Howie, former president of the British Orthopaedic Association (BOA), said the modular neck products had a “higher failure rate… because the neck stem junction fails more quickly and causes more wear”.
He said the idea behind it was to tailor “various parameters of the neck” to fit the patient, but “in fact, it wasn’t all that successful”.
“If you’ve got three joints, then you’ll get three times the wear problem. And not only that, but one of the joints is a metal on metal joint, and if it doesn’t fit correctly, you’ll get lots of small wear particles,” he said.
“The actual chromium and cobalt isn’t the problem. It’s how it’s used that’s the problem, and the number of bearing surfaces that you’ve got. The ions are not really produced unless they are worn in a certain way.”
Studies have linked the implants to higher than usual failure rates, fractures and poisoning.
Experts at Thomas Jefferson University Hospital in Philadelphia found the implants needed replacing within two years in 13 per cent of patients, and within five years in 22 per cent.
Last year, a study led by the University of Strathclyde in Glasgow found “there is now very strong evidence of a link between hip replacements where metals such as cobalt and chromium are used in the artificial joint bearings, and the possibility of developing heart complications”.
Others have also suffered the effects of cobalt poisoning. Howard Piper was one such patient who has successfully been compensated by the NHS for clinical negligence after cobalt poisoning left him with fatigue, mood swings and sight loss.
Dennis Reed, director of over-60s campaign group Silver Voices, called on the NHS and MHRA to investigate what could be “a big scandal” and “not just put their finger in the air and hope”.
“We need to know the extent of the problems with these hips and what dangers the patients concerned are facing,” he said. “The NHS should investigate and report back urgently on the number of patients who have received these hips and over what period, how many have failed so far, and how many patients have suffered symptoms of cobalt poisoning to date.
“All patients potentially affected should be contacted and their current health conditions assessed, with a hip replacement option if it proves necessary.”
Paul Whiteing, the chief executive of patient safety charity Action Against Medical Accidents, said: “Patients need to have confidence that the regulators responsible for licensing these products are satisfied as to their safety and that where concerns do arise there are effective mechanisms for contacting affected patients and, without alarming them, draw to their attention the symptoms that may arise and what to do if they do.”
In the US the crisis has already been laid bare, with lawsuits leading to compensation despite manufacturers not accepting liability.
In one case Robert Rembisz, a 75-year-old from Florida, is suing a manufacturer, alleging that the corrosion of the product caused elevated metal levels in his blood and “neurologic symptoms” including nerve damage, tinnitus, and balance and coordination problems.
“The problems I developed weren’t even close to my hip,” Rembisz said. “This problem could be occurring in [other people’s] bodies as well and they don’t even know it.”
He claimed his cobalt levels were peaking at nearly 12 times the normal range.
The failing cobalt hips have even been used as storylines in the medical TV dramas Grey’s Anatomy, General Hospital and House.
Mrs O’Neill’s blood work showed her cobalt levels were seven times the normal range at one point. The 56-year-old now runs a WhatsApp group formed of fellow patients who attended psychotherapy sessions together to offer help and support.
“I live my life like today is my last day, and I never stop helping other people, because for me this is a form of healing, but I will never, ever come to terms with what happened unless it is recognized. If I get recognition for what’s happened, I can then accept it and move on with the life I have left,” she said.
 Martyn Porter, former president of the BOA, urged caution, stating that the “vast majority of patients with cobalt chrome in them are going to be absolutely fine” and there were guidelines in place for “the patients who are at risk” for hospitals to follow.
Martyn Porter, former president of the BOA, urged caution, stating that the “vast majority of patients with cobalt chrome in them are going to be absolutely fine” and there were guidelines in place for “the patients who are at risk” for hospitals to follow.“Theoretically, there is a slight possibility that even with very low release of cobalt, over many, many years, it could have an effect, and some surgeons now say, because of that potential risk, ‘I’d rather just not use cobalt chrome’,” he said. “So there’s a massive increase in using ceramic femoral heads, particularly in the United States and even in the UK.”
But he added: “I don’t think the evidence is strong enough to say we should stop using cobalt chrome completely.”
Janine Jolly, deputy director, benefit risk evaluation at the MHRA, said: “We monitor the safety of all hip replacement devices to ensure the benefit risk balance remains favourable.
We rigorously review all adverse events reported to us, as well as published literature and other data sources such as the National Joint Registry (NJR). We also regularly seek advice from experts in the field and will continue to review emerging evidence, taking appropriate action if further risks are identified.”
A spokesman for UHCW said: “We are sorry to hear about Ms O’Neill’s ongoing difficulties. When she had her hip replacement operation at the Hospital of St Cross, Rugby in 2014, the implant was in general use in the NHS and there was no indication of any significant issues with the product.
“Although in 2014 our practice was to follow-up most of our patients every year or two, this wasn’t mandatory for her type of hip replacement. Complications with the implants are rare and had Ms O’Neill continued to be seen in our outpatients beyond 2017, then we could potentially have established the source of her ongoing symptoms.”
An NHS spokesman said: “The safety of medical devices is regulated by the MHRA, with the NHS supporting its responses to any relevant risks to keep patients safe.”